Q&A with Aaron Scott
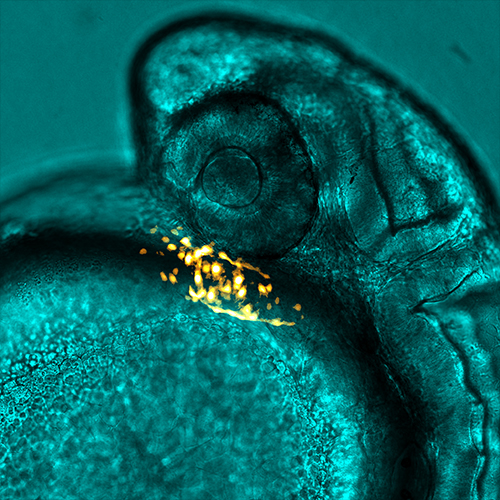
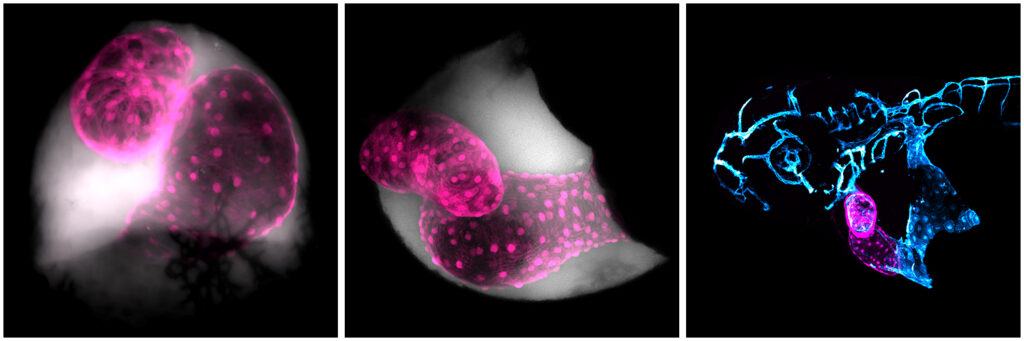
Picture this: fluorescent green cells dancing inside a fluorescent red pulsing shape, the heart of a zebrafish. It looks an awful lot like a baby alien. And it’s all happening beneath a microscope lens. Peering into this world, you suddenly and simultaneously feel so big and so small in comparison.

This is an everyday experience for Aaron Scott, although you’ll see the magic isn’t lost on him. As an in vivo cell biologist at the University of Bristol he uses microscopy to visualize nano-sized extracellular vesicles in zebrafish to understand their connection to cardiovascular disease.
Aaron will be judging the From the Lab category for the 2021 Visualizing Science Contest.
What drew you to cell biology?
I took a rather winding path: starting with pure biology, quickly moving to zoology, a small stint with environmental biology and finally landing on in vivo cell biology. I’ve always loved animals, but to my disbelief zoology as an undergraduate left me rather uninspired. I then took a sidestep into environmental biology and after joining an awesome lab at the interface between ecotoxicology and cell biology, I started working with transgenic zebrafish and fell in love with cell biology. The first time I looked down a microscope and to see a fluorescent heart beating, I was hooked! So, the short answer is, transgenic zebrafish and microscopes—6 years later and I am just as fascinated by this part of my job.
Every time you look down the microscope, you’re guaranteed to see something new and exciting—I particularly love the novelty of looking at transgenic lines I’ve never used before. There’s just so much power in the light microscopes we use and when you’ve got fluorescently labelled cells going about their business inside a living organism that is transparent, there’s a whole world to explore, a world I’d never dreamt was even accessible when I started my journey into science.