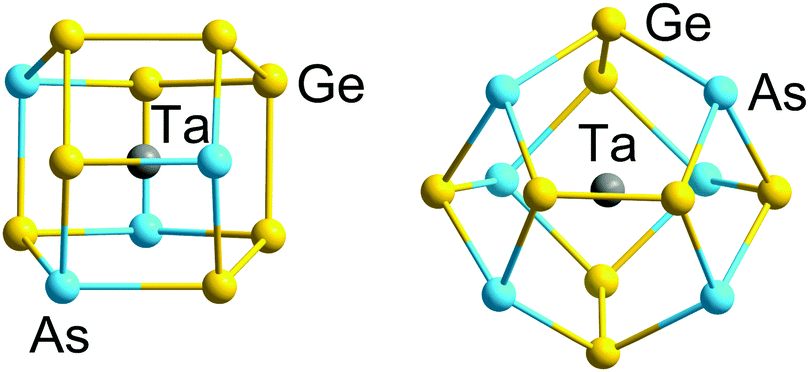
Despite their complexity, clusters are “atomically precise nano-objects.” With a lot of work, they can be reproduced and used reliably for creating chemical reactions with a wide variety of uses. For example, Dehnen and colleagues have developed an inorganic cluster that could be used to make cheap and sustainable white lights.
For Dehnen, two unanswered questions in inorganic cluster chemistry stand out.
First, “… how [do] metal clusters or non-metal-bridged metal clusters form?”. Understanding this formation, Dehnen notes, could be hugely beneficial because it would mean researchers would be “in a position to predict, design, and control the synthesis of clusters much better than we can do this to date, which would save time and resources.”
Second, on Dehnen’s list: What’s really going on with the catalytic activity (the rate of a chemical reaction) of cluster materials?
“There is a lot of evidence for catalytic activity of cluster materials and also for a variety of further useful properties, like supercontinuum generation [when a laser beam travels through a material and then splits into multiple different colours],” Dehnen explains. “However, most of this is only fragmentarily explored or understood, so we need much more knowledge in order to be able to ultimately apply the compounds.”
Stay tuned for chemical queries about nanocrystal synthesis and chemical probes in part II of this series.